Welcome to the Section of Structural & Synthetic Biology at Imperial College London
The Section of Structural & Synthetic Biology aims to bridge the gap between fundamental biophysics and medical research into the mechanisms underlying human diseases. We are situated in South Kensington, as part of the Department of Infectious Disease at Imperial College London.
Research groups:
Introduction to The Section:
The Section for Structural Biology within the Department of Medicine was set up in 2014 in order to integrate fundamental structural and molecular biology directly with medical research at Imperial College London. Prof. Paul Freemont heads up the section, which also includes the groups of Prof. Xiaodong Zhang, Prof. Dale Wigley, Dr Harry Low, Dr. Chris Aylett and Dr David Riglar at present, with more scientists on the way. Our research focuses on understanding the molecular mechanisms underlying factors linked to human disease, using a multi-disciplinary approach to dissect their structure and function in parallel. The interests of the investigators within the section span many fundamental cellular processes including chromatin remodelling, DNA replication, transcription, the repair of DNA damage, membrane fusion and protein degradation, and signal transduction, all of which are linked to important human diseases. Our aim is to make the most direct contributions possible to understanding these conditions with the benefit of modern techniques.The SSB is an exciting and rewarding place to work, providing a hub of high-level knowledge bearing on the fundamentals of biochemistry and structural biology, right in the heart of one of the world’s most important and vibrant cities. We are happy to hear from any researchers interested in asking about our work, or thinking about coming to join us, and to share our techniques and expertise with collaborators both in the UK and abroad.
Molecular mechanism - key to clinical progress
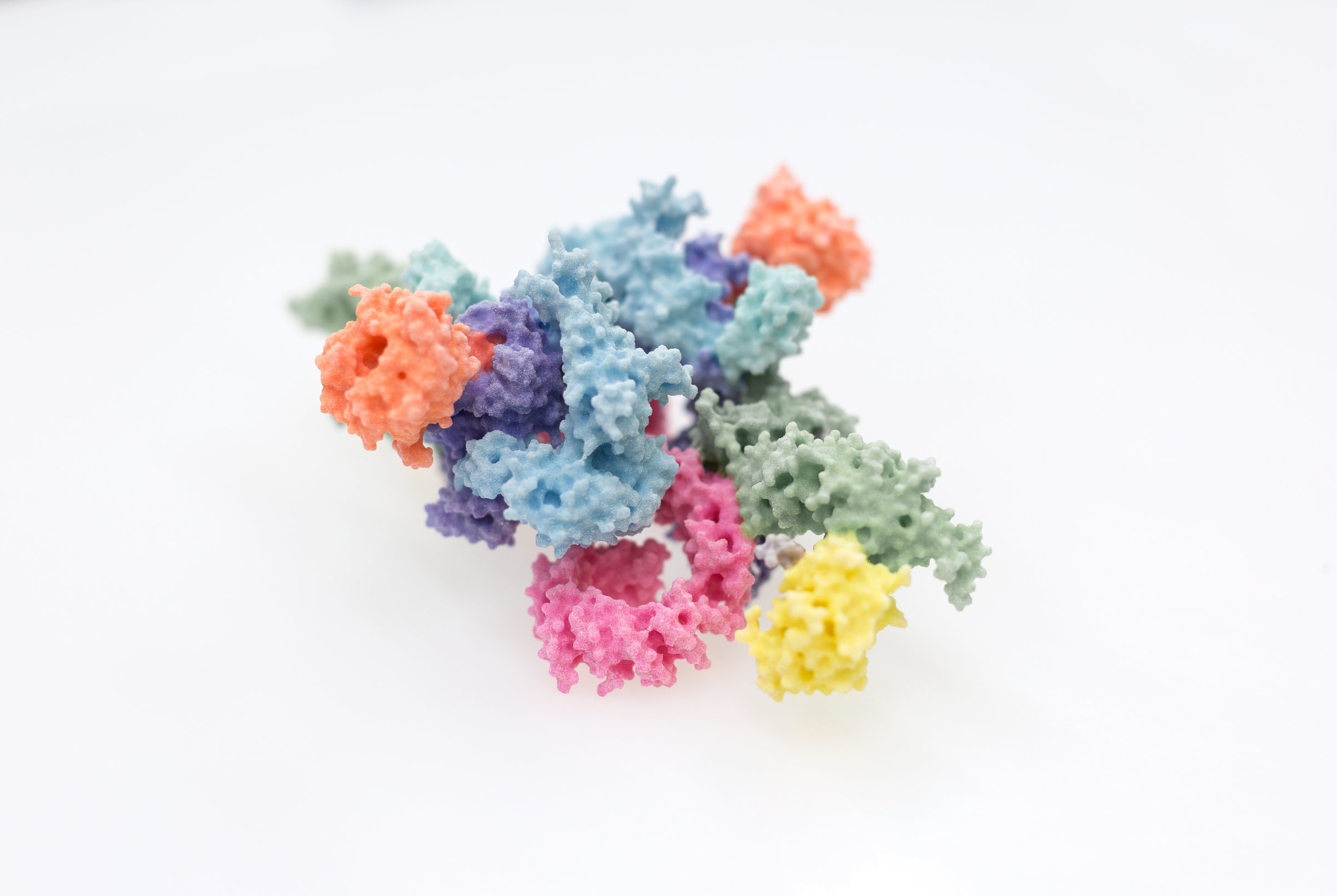
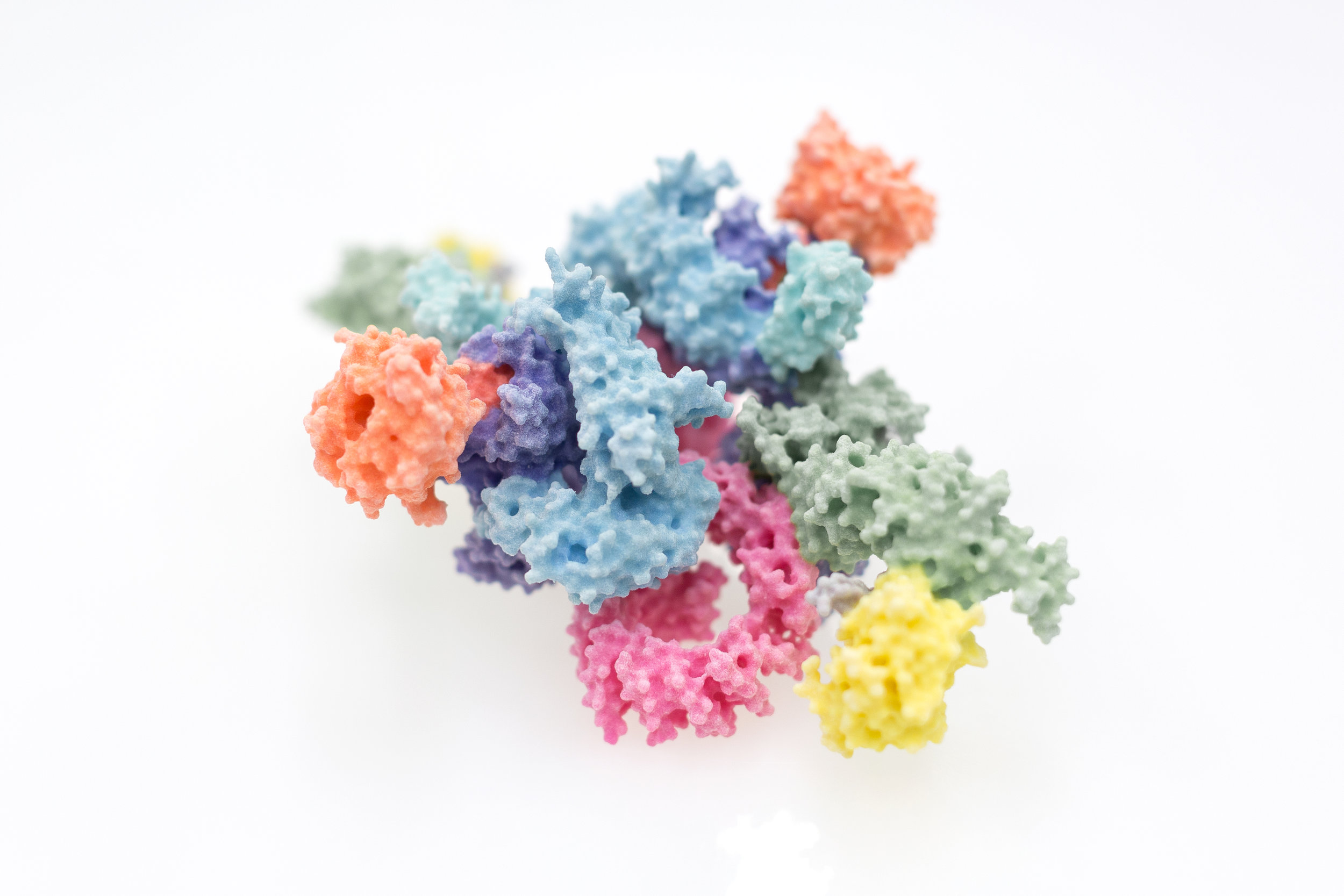
Since the key molecules of life, DNA, RNA and protein, were understood in the 1950s, the molecular mechanisms underlying almost every aspect of biology have started to become incrementally clearer and clearer. We now have catalogues and blueprints, such as proteomes and genome sequences, for not just humans, but many of the most important disease-causing bacteria and viruses. Interpreting this wealth of data remains a fraught process however, the exact way in which molecules mesh to form the machinery of the cell requires their visualisation in atomic detail, which is still a difficult and costly endeavour. This process has had new life breathed into it over the past few years through great strides forward in the technique of electron cryo-microscopy, for which the Nobel prize for Chemistry was awarded to Dubochet, Frank and Henderson in 2017. Scientists within the Section have been intimately involved in the process of understanding proteins involved in immunology, cancer, and DNA damage repair through such novel approaches. Given the efforts required to eke such knowledge out of the molecules of life themselves, and the great potential for new treatments, new drugs or new approaches entirely, we are keen to share our work as widely and with as much impact as possible.
Mind the gap - a multi-disciplinary approach to medical research
The Section for Structural Biology was founded by Paul and Xiaodong, both of whom have spent their careers committed to producing scientific results that have real world applications. Having both seen many important mechanistic results founder in the gap between the laboratory bench and bedside, they reasoned that in order to continue to have a real impact through medical research, they would need to integrate directly into a medical centre of excellence. The medical department at Imperial College is at the forefront of clinical research in the UK, and they therefore established the Section within the Department of Medicine at Imperial. The focus of work within the Section for Structural Biology is to build understanding of disease mechanisms from the mechanistic level, looking at individual molecules, to cellular models and eventually clinical applications. This commitment has not only been to approach diseases of the Western World, as scientists within the section are also directly pursuing collaborations aiming to aid developing nations. Paul and Xiaodong are recruiting like-minded scientists, with impressive research track records and a similar shared interest in human disease mechanisms, expanding to tackle new problems.
Help us make a difference - collaboration and expansion
The scientists overseeing the Section are constantly looking for opportunities to expand both our collaborative network and our team here. We are on the lookout for like-minded scientists who wish to make a difference, and who are at any stage in their careers. In particular, scientists with a clinical or pharmacological bent would be welcomed with open arms; the Section remains a work in progress, and our commitment to promote collaboration and integration between structural and mechanistic understanding and the cellular and clinical aspects of medicine cannot make progress without active, prolonged engagement from both sides.
Follow our latest news on Twitter:
-
RT @PaulFreemont: We have two exciting academic positions available for ambitious researchers in structural biology and synthetic bio… https://t.co/5q6MsBWzlh
-
RT @rkelwick: Our research is now featured on @GrowKudos https://t.co/LUESD1ceS2 @PaulFreemont @ajwebb1979 #globalhealth #synbio #DNAbiosensor
-
RT @driglar: We are seeking a research assistant to join our team (https://t.co/KeYJtOrwKq) at @ImperialInfect for an exciting… https://t.co/IGT36mnJXE